
Combining its highly sensitive assay with ASTX™, Resolve mdx helps providers select the right treatment for patients with UTI symptoms. Personalized antibiotic options delivered within 48 hours. Direct UTI treatment quickly with Resolve mdx.
How Resolve mdx works
Resolve mdx combines the precision of PCR for pathogen identification and resistance gene detection with proprietary susceptibility testing, called ASTXTM, for personalized antimicrobial solutions to address your patient’s specific infection.

Key Features and Benefits
Test Details: UTI and STI Test
STI Testing Available for Additional Results
The Resolve mdx STI test detects 8 pathogens, including Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. The highly sensitive assay detects STI pathogens to as low as 103. Results are reported within 24-48 hours from receipt at lab.
UTI Test
BACTERIA:
- Acinetobacter baumannii
- Actinotignum schaalii
- Citrobacter freundii
- Citrobacter koseri
- Enterobacter cloacae
- Enterococcus faecalis
- Enterococcus faecium
- Escherichia coli
- Gardnerella vaginalis
- Klebsiella aerogenes
- Klebsiella oxytoca
- Klebsiella pneumoniae
- Morganella morganii
- Mycoplasma genitalium
- Mycoplasma hominis
- Proteus mirabilis
- Pseudomonas aeruginosa
- Serratia marcescens
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus saprophyticus
- Streptococcus agalactiae
- Streptococcus pyogenes
- Ureaplasma parvum
- Ureaplasma urealyticum
YEAST:
- Candida albicans
- Candida auris
- Candida glabrata
- Candida krusei
- Candida parapsilosis
STI Test
- Gardnerella vaginalis
- Mycoplasma genitalium
- Mycoplasma hominis
- Ureaplasma parvum
- Ureaplasma urealyticum
- Chlamydia trachomatis
- Neisseria gonorrhoeae
- Trichomonas vaginalis
Resistance Gene Groups
- Carbapenem (CRE) Resistant
- Extended Spectrum
- Beta-Lactamase (ESBL)
- Fluoroquinolone
- Methicillin Resistance (mecA)
- Mobilized Colistin Resistance
- Trimethoprim/Sulfamethoxazole
- Vancomycin Resistant (VRE)
How to Order Resolve mdx
Ordering Resolve mdx for Urinary Tract
Infection is simple and easy.
Physician Portal
Secure mdx is a safe and secure HIPAA compliant tool that gives you access to patient reports online.
Polypharmacy solutions for
complicated cases
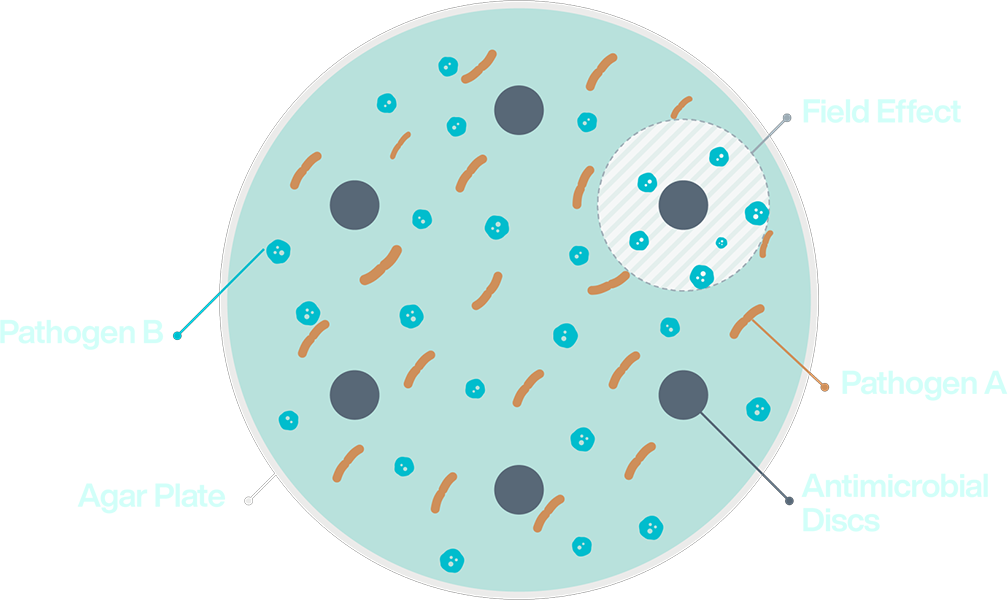
In polymicrobial samples, pathogens may demonstrate different antimicrobial susceptibilities, creating a “field effect” in the culture. In these cases, a single oral antimicrobial solution will not be effective against the infection. Resolve mdx uses ASTX™ to detect the subpopulation of resistant bacteria in the sample and identify oral antimicrobials to address each pathogen.
Actionable Report
Resolve mdx facilitates diagnostic decision making with an actionable and easy to interpret report with the information needed to make informed treatment decisions.
- Organisms identified
- Resistance genes detected
- Whole urine susceptibility results
- Prioritized oral antimicrobial options
Case Studies
Resolve mdx positive: polymicrobial case study.
- Female, age: 68
- History of complicated UTIs
- 2 allergies: Contrast dye, Doxycycline
- Previous treatment failures
Antibiotic Stewardship
A recent study found that nearly 50% of UTI patients received the wrong antibiotic.2 By identifying patient-specific treatment options, Resolve mdx promotes appropriate use of antibiotics, reducing reliance on empiric therapy. Resolve mdx tests uropathogens against a panel of 26 antibiotics. Except in rare cases, Resolve mdx provides at least one (1) oral antimicrobial option to resolve each patient’s infection.
References
1 Vollstedt A, Baunoch D, Wojno KJ, Luke N, Cline K, et al. (2020). Multisite Prospective Comparison of Multiplex Polymerase Chain Reaction Testing with Urine Culture for Diagnosis of Urinary Tract Infections in Symptomatic Patients. J Sur urology, JSU-102. DOI: 10.29011/ JSU-102.100002.
2 Clark AW, Durkin MJ, Olsen MA, et al. Rural–urban differences in antibiotic prescribing for uncomplicated urinary tract infection. Infection Control & Hospital Epidemiology.2021;42(12):1437-1444. doi:10.1017/ice.2021.21

Resolve mdx UTI and STI testing results from the comfort of home
Sometimes it’s difficult to see a patient for an in-office visit. With our Resolve mdx At-Home program, patients can submit a sample for Resolve mdx UTI and STI testing from the comfort of their home. Once a provider places an order on our Portal, our mdxhealth Xperts will take care of everything else. Collecting the urine sample is easy. Click to see how.




















