Clinical Evidence
Measure what matters in your low-risk patients
The Genomic Prostate Score (formerly Oncotype DX GPS) test includes the most robust definition of adverse pathology, which is a strong predictor of long-term endpoints.1
A universal endpoint
Standard risk assessment tools for localized prostate cancer, such as biopsy, imaging, and PSA, can aid in assessing a patient’s risk of harboring adverse pathology or its components.
A unique assay
The GPS test is the only genomic test to provide the risk of adverse pathology as well as the risks of its components (pT3a+ and Gleason ≥ 4+3).5
A useful definition
Extra prostatic extension (pT3a+ disease or higher) is included in the GPS assay’s definition of adverse pathology, which is important because it is a strong predictor of distant metastasis.2
Endpoints Data
The presence of adverse pathology at radical prostatectomy (RP) is a near-term endpoint that’s strongly prognostic for long-term outcomes.1
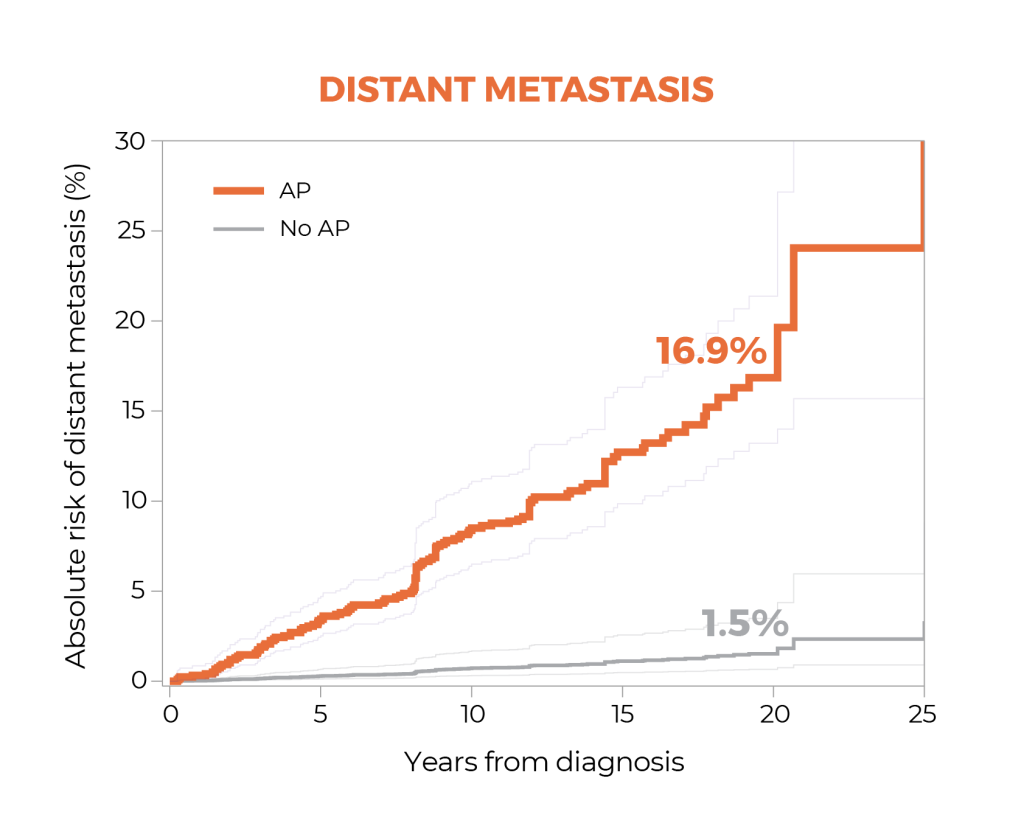
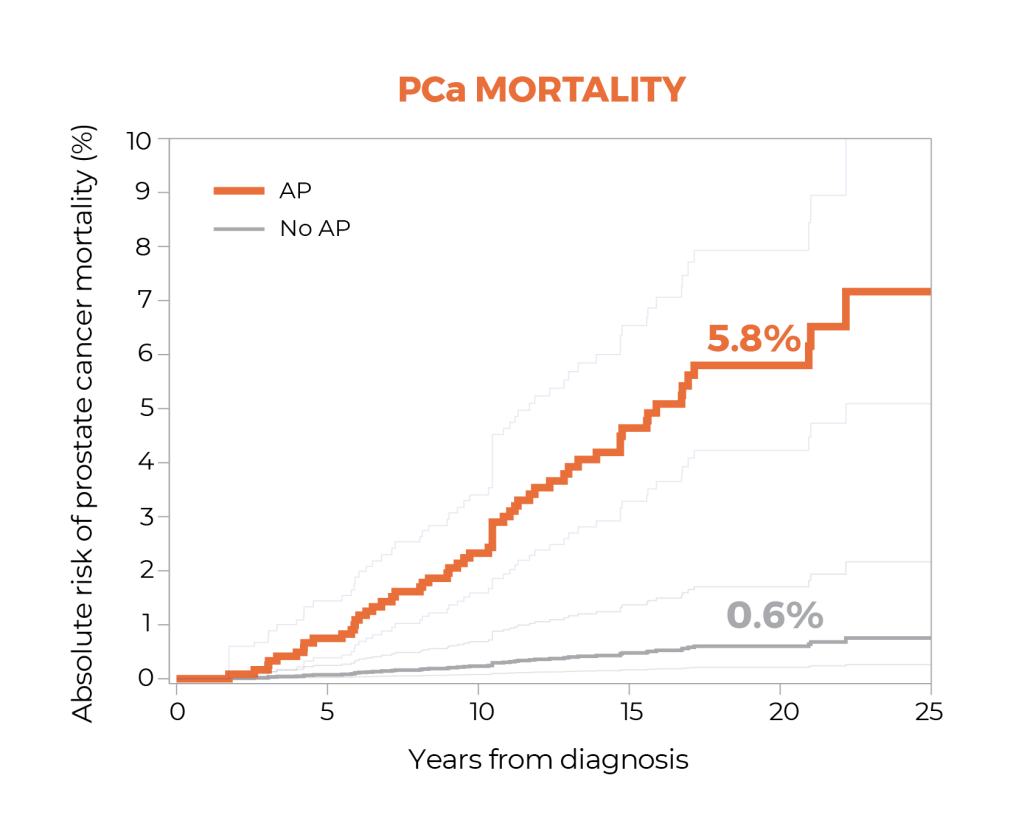
Outcomes Data
The GPS test was associated with up to 20-year outcomes.3
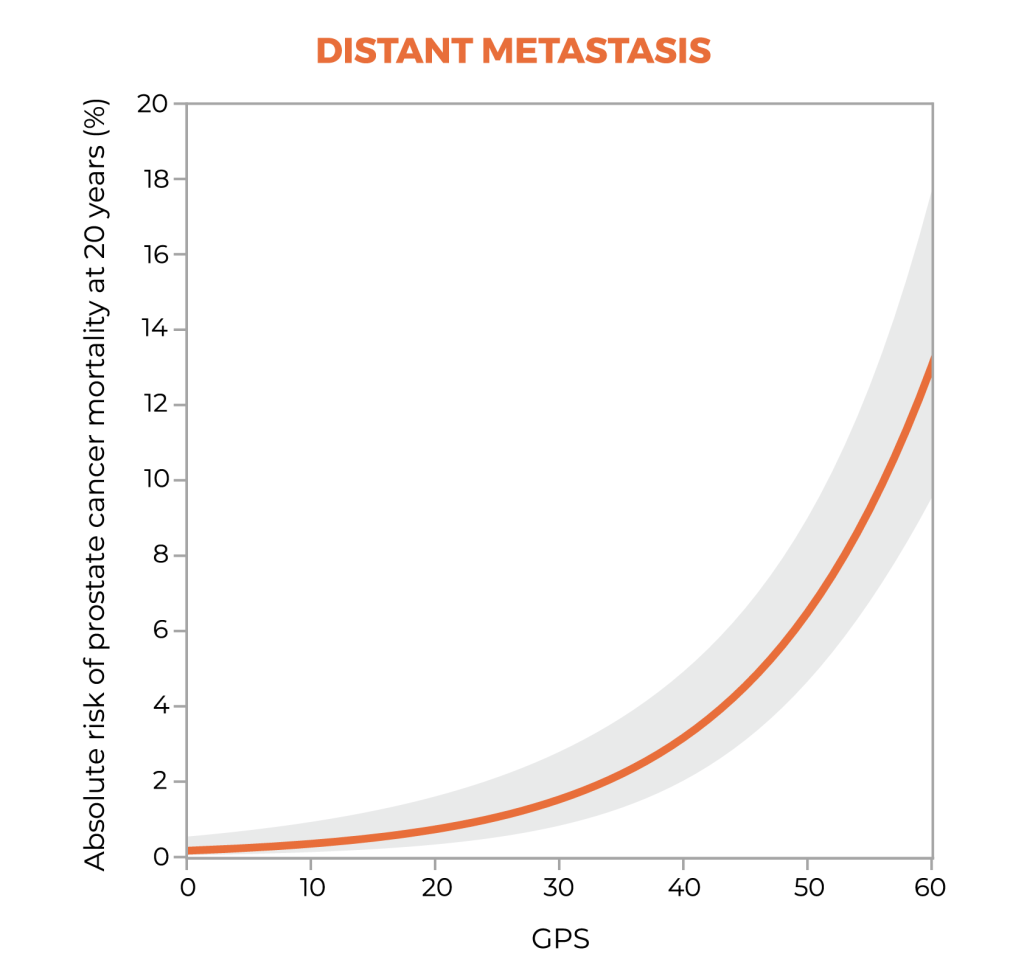
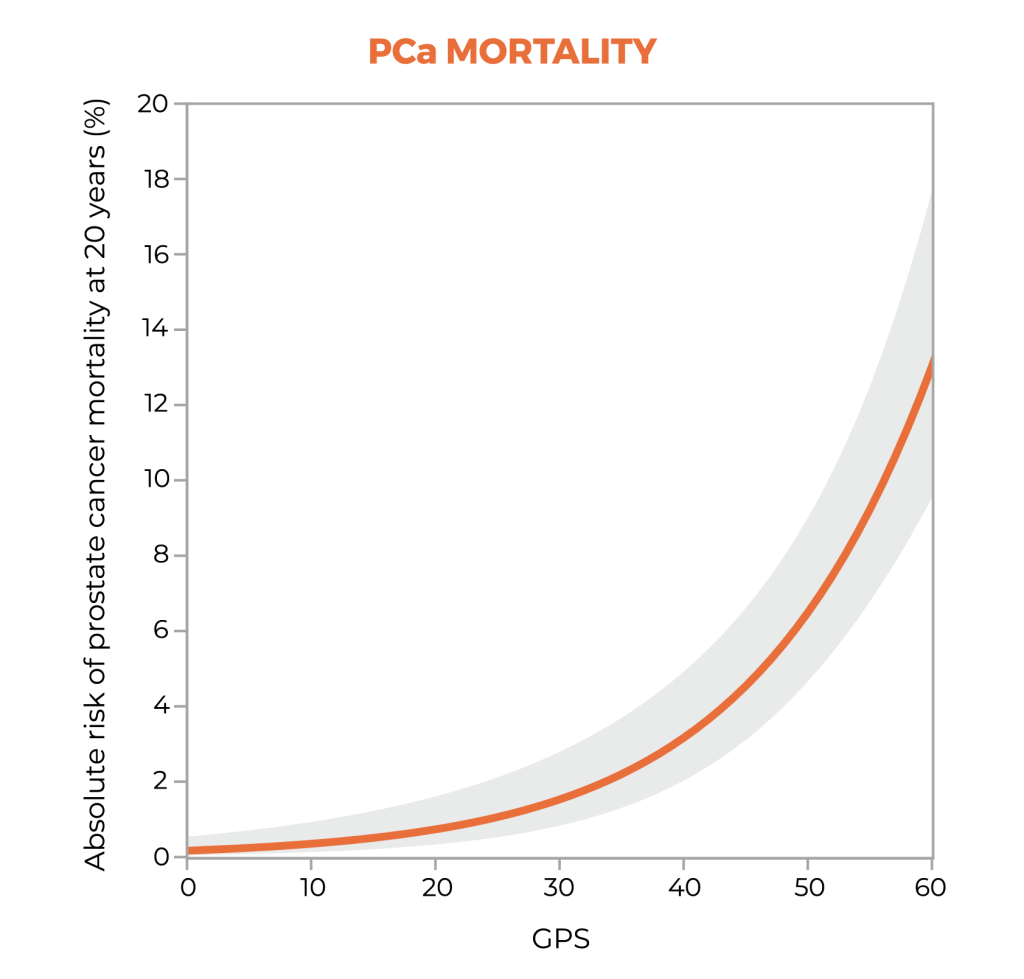
Long-term data show the clinical utility of stratifying risk with the GPS test.3
20-year outcomes1,3
A study suggests that the GPS™ test may be prognostic for both distant metastasis and prostate cancer-specific mortality through 20 years after diagnosis, and that the GPS test together with clinical factors may improve risk assessment over clinical factors alone.1
In a large cohort of men with largely clinically low-risk prostate cancer treated with radical prostatectomy, the study suggests the presence of adverse surgical pathology significantly predicted 20-year risk of metastasis and prostate cancer-specific mortality. Patients with adverse pathology at radical prostatectomy had a 12-fold higher risk of metastasis than those with favorable pathology, demonstrating the value of this endpoint for assessing risk in this population.1
Measure what matters in your high-risk patients
The GPS test is clinically validated to answer key questions in the high-risk setting with endpoints that guide decision making.4,5
PROVIDES prognostic information about a patient’s individual risk of death and metastasis within 10 years of RP4,5
The GPS test can help you determine the appropriate level of treatment by knowing the likelihood of disease progression.4,5
Endpoints Data
The GPS test provides a score that is strongly predictive of adverse outcomes in high-risk patients.4,5
New High Risk Patient Report is now available.
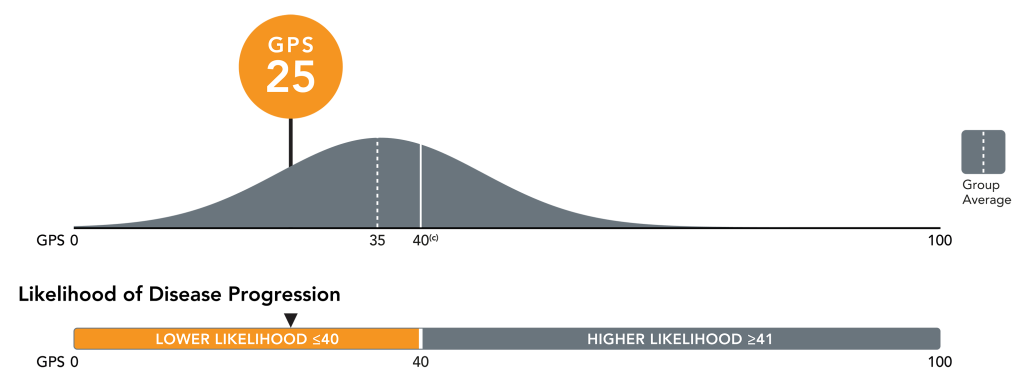
Precise risk assessment for informed treatment planning with the GPS cut point4
- For patients with lower risk levels (≤40), monotherapy or minimization of combination therapy may be sufficient
- For patients with higher risk levels (>40), a more aggressive therapy approach may be appropriate
Outcomes Data
The GPS cut point is validated to stratify patients into categorical risk groups above and below 40 that are strongly associated with disease progression in clinically high risk patients.4
- Patients with a GPS result of ≤40 had outcomes similar to those with favorable intermediate disease; patients with a result of >40 had outcomes resembling those with high-risk disease.4
Rigorous development and clinical validation
The GPS was developed in collaboration with the Cleveland Clinic and the University of California, San Francisco (UCSF) to specifically address the many challenges inherent to prostate cancer risk assessment.6
The genes composing the GPS test were chosen for their6:
- Correlation with tumor aggressiveness regardless of multifocality or heterogeneity
- Performance in biopsy samples with small tumor volume
Over 9000 patients have been included in development studies, clinical validation studies, and clinical utility studies.6-23
Clinical Validation Studies
The clinical validation studies for the GPS test were prospectively designed studies of archival tissue from a large, diverse set of data sources:
- Kaiser Permanente, Northern California (KPNC) (N=259)8
- University of California, San Francisco (UCSF) (N=395)6
- Center for Prostate Disease Research (CPDR) (N=402)7
A meta-analysis combining the UCSF and CPDR cohorts (N=732)24 provided more precise estimates for likelihood of adverse pathology.
See the benefits of integrating the test into your practice
The impact of the GPS test on treatment planning has been demonstrated in multiple clinical utility studies.11,12
Prospective study
Assessed changes in urologists’ treatment recommendations pre- and post- GPS test results. This decision-impact study demonstrated a 26% absolute change in management recommendations. Physician confidence improved in 85% of cases.11
Retrospective study
Compared 6-month management received in clinically low-risk patients with and without the GPS test results. This chart-review analysis revealed a 56% relative increase in the number of patients on active surveillance/watchful waiting at 6 months post-diagnosis when GPS testing was added to standard decision-making tools.12
- Gearman DJ, Morlacco A, Cheville JC, Rangel LJ, Karnes RJ. Comparison of Pathological and Oncologic Outcomes of Favorable Risk Gleason Score 3 + 4 and Low Risk Gleason Score 6 Prostate Cancer: Considerations for Active Surveillance. J Urol. 2018;199(5):1188-1195.
- Ellis EE and Frye TP. Role of multi-parametric magnetic resonance imaging fusion biopsy in active surveillance of prostate cancer: a systematic review. Ther Adv Urol. 2022;14:1-15.
- Guimaraes T, Gil M, Medeiros M, Andrade V, Guerra J, Pinheiro H, Fernandes F, et al. Magnetic resonance imaging target fusion biopsy vs. transrectal ultrasound-guided biopsy – A comparative study of ISUP score upgrading risk in the final radical prostatectomy specimen. Arch Ital Urol Androl. 2022; 94(3):278-284.
- Mehralivand S, Shih JH, Harmon S, et al. A grading system for the assessment of risk of extraprostatic extension of prostate cancer at multiparametric MRI. Radiology. 2019;290(3):709-719.
- Brooks MA, Thomas L, Magi-Galluzzi C, et al. GPS assay association with long-term cancer outcomes: twenty-year risk of distant metastasis and prostate cancer-specific mortality. JCO Precis Oncol. 2021;5:PO.20.00325.
















