Technology & Platform
Mdxhealth’s technology platform is called methylation-specific PCR (MSP), which is a DNA-based technology that functions on standard commercial PCR equipment. MSP is extremely powerful and accurate with the ability to detect a single cancer cell among 10,000 healthy cells in any type of bodily fluid or tissue. Mdxhealth has a broad portfolio of DNA methylation biomarkers targeted at individual genes that are used in its different products.
Mdxhealth Technology
Mdxhealth uses a molecular (gene-based) technology to improve cancer diagnosis and treatment. Individual genes (DNA biomarkers) in the human body can become modified in the presence of cancer. Mdxhealth has the ability to identify these modifications at the genetic level providing the physicians with a tool to aid in the diagnosis of cancer, assess the risk of recurrence (metastasis) of the cancer, and predict an individual patient’s likely response to cancer treatment.
DNA methylation is a valuable tool for assessing cancer because methylated DNA biomarkers occur in almost all malignancies. The importance of DNA methylation in cancer was discovered in the 1990s. Gene methylation is a control mechanism that regulates gene expression in DNA and occurs when a methyl group is added to one of the four building blocks of DNA, a cytosine. In several diseases, however, the promoter regions that carry the instructions to produce an essential protein can be over- or hypermethylated, effectively inhibiting protein production. Hypermethylation of genes, such as tumor suppressor genes, is associated with the presence and development of most cancers. And while changes in DNA methylation were initially thought to be the result of cancerous transformations, it is increasingly believed that it plays an active, causative role.
The pattern of gene hypermethylation in tumor cells is often specific to the tissue of origin and can be used to improve cancer detection, assess risk of recurrence, and predict a tumor’s response to therapy.
Methylation-Specific PCR (MSP)
Precise mapping of DNA methylation patterns in CpG islands has become essential for understanding diverse biological processes such as the regulation of imprinted genes, X chromosome inactivation, and tumor suppressor gene silencing in human cancer. Methylation-specific PCR can rapidly assess the methylation status of virtually any group of CpG sites within a CpG island, independent of the use of methylation-sensitive restriction enzymes. An MSP assay entails initial modification of DNA by sodium bisulfite, converting all unmethylated, but not methylated, cytosines to uracil, and subsequent amplification with primers specific for methylated versus unmethylated DNA. MSP requires only small quantities of DNA, is sensitive to 0.1% methylated alleles of a given CpG island locus, and can be performed on DNA extracted from paraffin-embedded samples. MSP eliminates the false positive results inherent to previous PCR-based approaches which relied on differential restriction enzyme cleavage to distinguish methylated from unmethylated DNA.
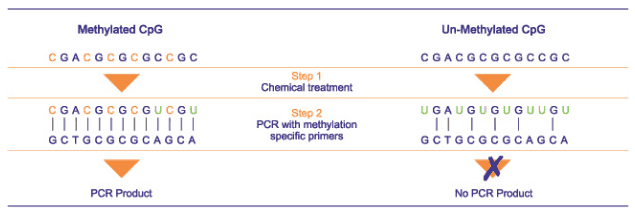
The use of a Real Time Quantitative MSP enables highly accurate discrimination between positive and negative findings.
PCR reactions can be multiplexed. This means that more than one reaction can be run simultaneously on the same sample in the same assay. This is accomplished by using a detector molecule that fluoresces at a different wavelength for each PCR reaction. By including primers for each marker of interest, along with detector molecules, which are also specific for each marker, a single sample can yield an independent result for each marker. This technique can increase the efficiency of the assay process in the laboratory, and allow detection of multiple methylated markers from a single patient sample.
MSP Platform for mdx
Mdxhealth has long recognized the importance and benefits of a platform-independent approach in the development and commercialization of methylation-based molecular diagnostic products. For this purpose, mdxhealth developed expertise on a wide variety of PCR platforms and has succeeded in developing assays on the most commonly used devices in clinical laboratories such as ABI Prism® 7900HT Fast-Realtime PCR system and Roche LightCycler® 480 System.
A wide variety of reagents are routinely assessed for assay development to ensure optimal assay performance and customization to meet the needs of our clients. These reagents include various fluorophores and quenchers combinations, TaqMan® probes, Amplifluor, Scorpions, molecular beacons in singleplex or multiplex and SybrGreen.
DNA preparation has also been thoroughly investigated, as DNA quality is essential for a successful diagnostic evaluation. Extraction methods such as magnetic-beads, silica columns and DNA precipitation are routinely used in a large range of sample types such as FFPE or fresh frozen tissue, urine, plasma or serum, sputum, broncho-alveolar lavages and stool.
Additionally, the company has demonstrated efficiency with excellent turn-around time, ranging from providing physicians timely results for patient treatment selection to large clinical studies where high throughput testing on thousands of samples and reports meeting ISO 9001 specifications is required.
Mdxhealth’s expertise in the development and validation of highly customized assays provides partners with a powerful and reliable tool to accelerate their own R&D programs.
MSP Assay Process
The MSP assays are laboratory developed tests performed on DNA isolated from patient samples. The extracted DNA is chemically treated to convert the unmethylated cytosines to uracil and subsequent PCR amplification with methylation specific primers allows for the quantitative measurement of methylated alleles. These results are subsequently used to report a clinically meaningful result to treating physicians.
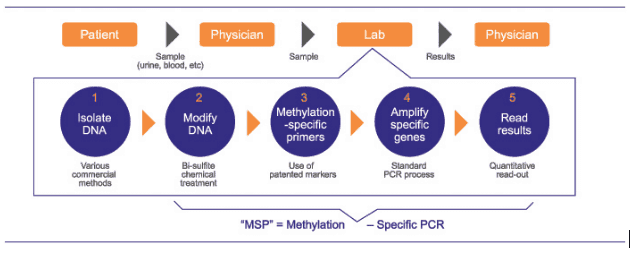
Summary of MSP technology
- Methylation present in all cancers: Methylation regulates gene expression|
- Specific genes methylated in each cancer: Abnormal methylation of specific genes is correlated with cancer development
- Sensitive detection in all sample types: Detects 1 cancer cell among 10,000 normal cells
- One or few genes suffice for tests: No complicated algorithms
- Technology performs on multiple PCR platforms: Commercially available equipment
- High-throughput testing: automated platforms available
Intellectual Property
Mdxhealth believes that our patent portfolio places the Company in a competitive position in the realm of molecular cancer diagnostics. We own or hold exclusive rights to a range of issued and pending patents in multiple countries worldwide covering our epigenetic and molecular tests and associated biomarkers and their methods of use. Many of our commercially important epigenetic technology and inventions are in-licensed from academic and commercial collaborators, including Johns Hopkins University.
Through our internal R&D programs, together with our NXTGNT (Epi)genomics research joint-venture with University of Gent and other academic and commercial collaborations, mdxhealth continues to be at the forefront of researching and understanding the link between cancer and methylation, and how this link can be translated into meaningful clinical and pharmaco molecular diagnostic products and services. We consider patent protection of the technologies, on which our products are based, to be a key factor to our success. Our intellectual property portfolio is managed by an in-house intellectual property team, which works in close collaboration with qualified external patent attorneys both in Europe and the United States.

















